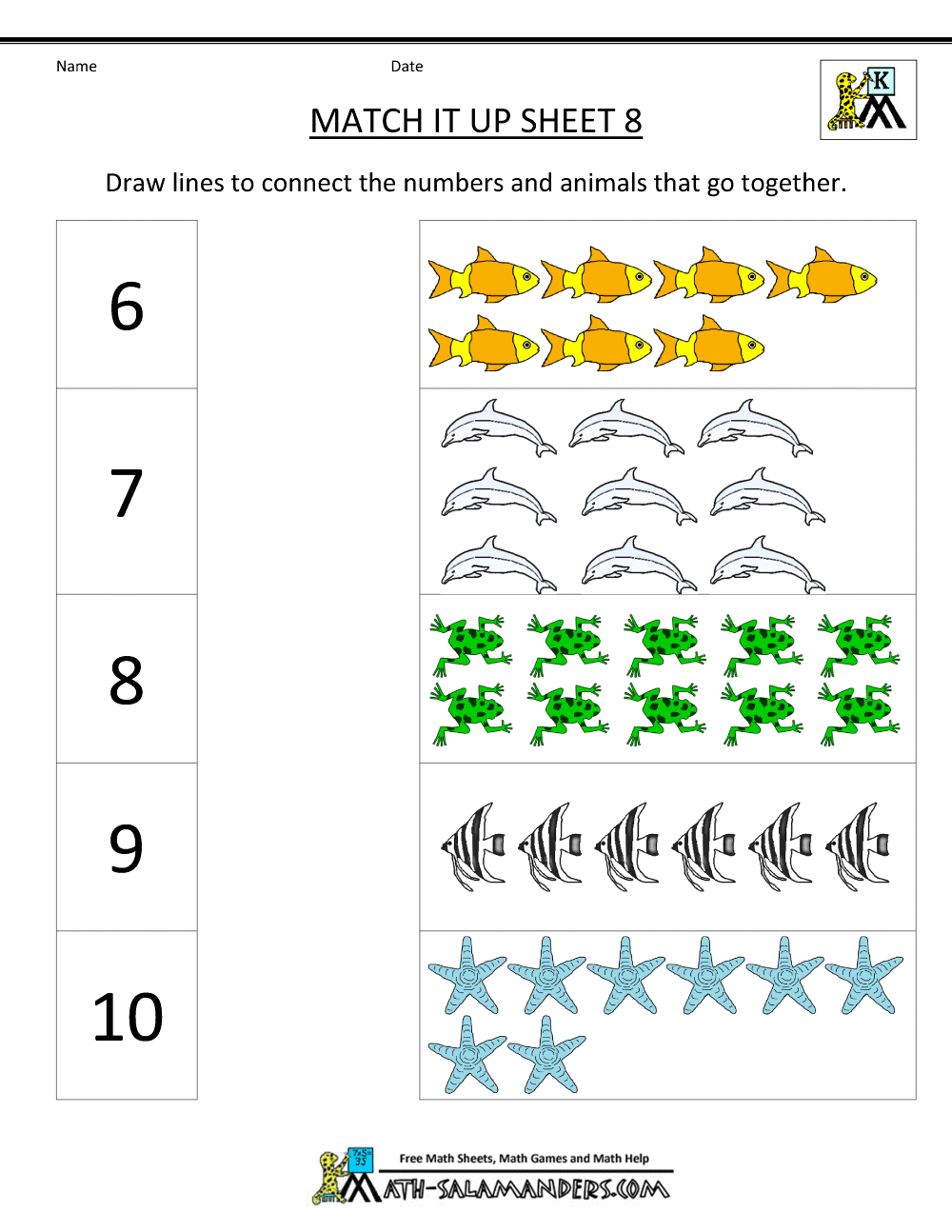
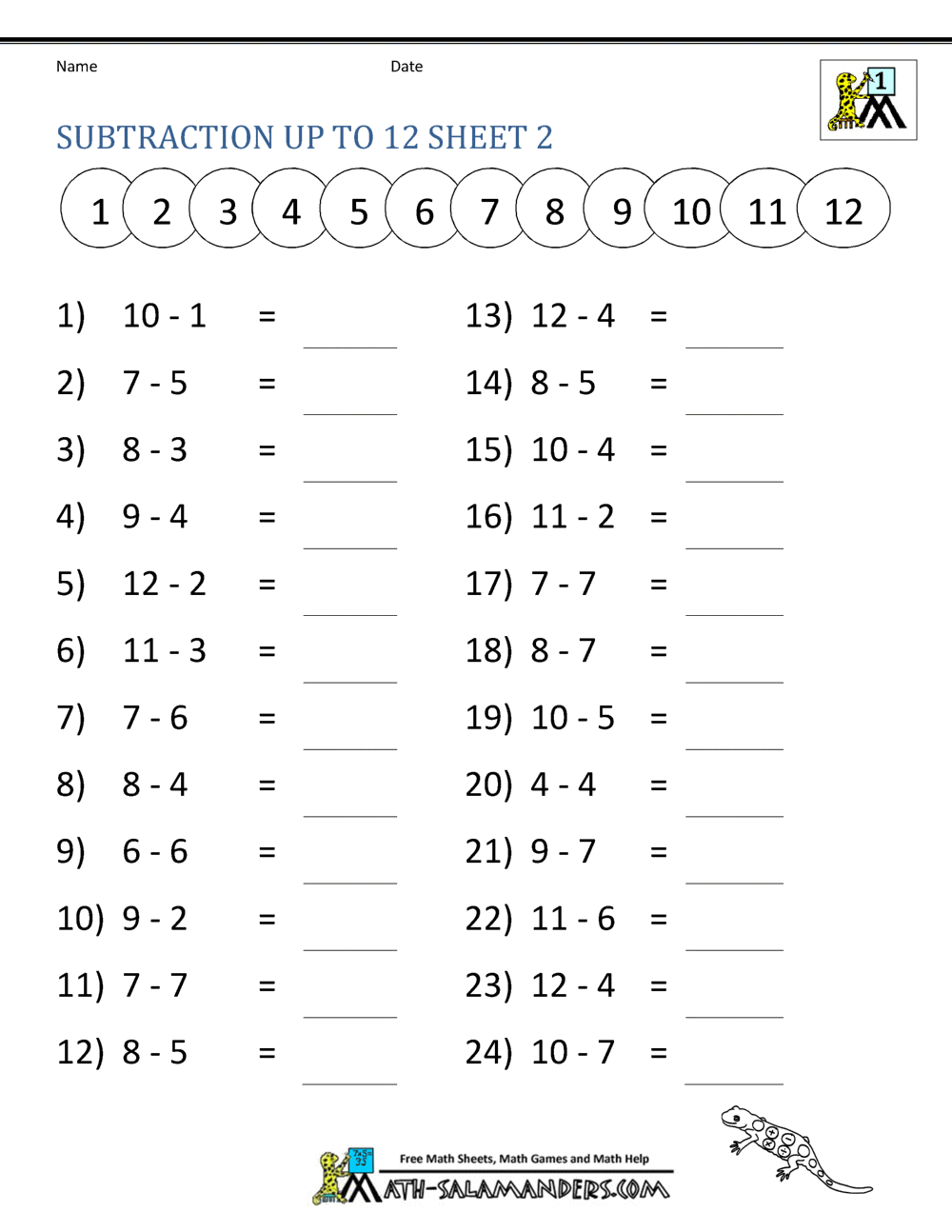
Solubility Product Constant Worksheet . Ksp=3×10 −5 5) the solubility of silver sulfide (ag. Write the solubility product constant expression for solid.
Short Answer Grade 12 Chemistry Solubility Product Constant, Ksp from www.tes.com Web 4) the solubility of calcium hydroxide (ca(oh) 2 ) is 0.02 mol/l. A solute is soluble if more than 1 g dissolves in 100 ml of. Write the solubility product constant expression for solid.
Source: www.tes.com
Write the solubility product constant expression for solid. Write the solubility product constant expression for solid lead (ii) chloride.
Source: briefencounters.ca
Web the solubility product is the ksp which is the product of the ions raised to their coefficients. A solute is soluble if more than 1 g dissolves in 100 ml of.
Source: goalkeepingintelligence.com
Web the solubility product is the ksp which is the product of the ions raised to their coefficients. Web 4) the solubility of calcium hydroxide (ca(oh) 2 ) is 0.02 mol/l.
Source: www.coursehero.com
Web fourth, substitute the equilibrium concentrations into the equilibrium expression and solve for k sp.; Web the solubility product ( ksp) is used to calculate equilibrium concentrations of the ions in solution, whereas the ion product ( q) describes concentrations that are not.
Source: www.liveworksheets.com
Web the solubility product is the ksp which is the product of the ions raised to their coefficients. For example, when we say the solubility product ( ksp) of caf 2 is 3.9 x 10.
Source: www.pinterest.com
For example, when we say the solubility product ( ksp) of caf 2 is 3.9 x 10. Web fourth, substitute the equilibrium concentrations into the equilibrium expression and solve for k sp.;
Source: studylib.net
Calculate the solubility product constant for this substance. 1) what is the concentration of a saturated silver (i) acetate solution?